Top Stories
The draft guidance states information concerning a clinical study of a biosimilar should only be included if it is necessary to demonstrate the safety and efficacy of the drug.
/ read more /
Adheren relies on chemical modifications, rather than genetic engineering, to create its cell-based immunotherapies.
/ read more / |
|
|
Subscribe
Subscribers can enjoy each full issue of BioPharm International in print, or via BioPharm apps.
subscription offers |
 |
|
|
|
ADVERTISEMENT
 |
|
|
Industry News
Although many immunotherapies focus on tumor-associated antigens, many of the peptide targets on the surface of cells have not be validated.
/ read more /
Solutions to circumvent heterogenous mixtures of antibody-drug conjugates and to ensure uniform drug-to-antibody ratio were discussed at a recent Catalent Applied Drug Delivery Institute symposium.
/ read more /
More Industry News
|
|
|
Biopharma News
A team of Bristol-Myers Squibb scientists will work in a new laboratory at the National Institute of Bioprocessing Research and Training facility in Dublin, Ireland.
/ read more /
The new research scheme will be based in GE's Turkey-based innovation center, and will help support the region's biopharmaceutical industry.
/ read more /
More Biopharma News
|
|
|
ADVERTISEMENT
 |
|
|
Supplier News
The companies will merge to create a gene therapy biologics CDMO.
/ read more /
The companies will provide assays for functional comparability, for use in quality control, lot release, and stability testing of biosimilars and biobetters.
/ read more /
More Supplier News
|
|
|
Regulatory News
The drug is marketed by Eli Lilly in the US, and will be available at the beginning of the second quarter of 2016.
/ read more /
Cinqair is approved for patients that have a history of severe asthma attacks despite receiving their current asthma medication.
/ read more /
More Regulatory News
|
|
|
|
FEATURED TOPICS |
|
GLOBAL REPORT
Aging populations and increased access to healthcare translates into opportunities for biopharmaceutical companies. JLL's 2015 Life Sciences Outlook report highlights global clusters worth watching.
/ read more /
ANALYTICAL BEST PRACTICES
This article defines the concept, justification, and method of removal of out-of-trend points in stability modelling and shelf-life prediction.
/ read more /
|
|
|
|
|
FILL/FINISH
Two experts discuss best practices to achieve acceptable sterility assurance levels for aseptically filled products.
/ read more /
FORMULATION
In this article, the author reviews some of the techniques that can yield valuable information on protein stability, focusing specifically on protein aggregation. Emphasis is placed on the enhanced information made available when technologies are used orthogonally, and the alignment of different approaches with specific stages of the biopharmaceutical development workflow.
/ read more /
|
|
|
KEYNOTE SERIES AT INTERPHEX 2016
At INTERPHEX 2016, Pharmaceutical Technology and BioPharm International are sponsoring a Keynote Series addressing leading bio/pharma industry issues.
In a panel discussion, Industry experts will discuss how technology advances are addressing challenges in biopharmaceutical development including quality control of raw materials, implementation of single-use technologies, process monitoring, and downstream purification.
Tuesday, April 26, 10:15 am to 11:45 am
Learn more
How will consolidation in the bio/pharmaceutical and contract services market, a changing financial market, and an active political and regulatory year shape the fortunes of the contract services market? In his annual presentation, industry expert Jim Miller will offer his perspectives on the contract services landscape for the next few years.
Wednesday, April 27, 10:30 am – 11:30 am
Learn more
Sterile injectables have been in extremely short supply, and industry efforts have been focusing on root causes involving infrastructure, quality, and efficiency. Experts involved in this work discuss recent initiatives, and offer insights into what must be done to prevent injectables shortages in the future.
Wednesday, April 27, 3:30 – 4:30 pm
Learn more
|
Webcasts
On Demand
Understanding the disulfide bonds (DSBs) in biotherapeutics is required for verifying its structure and evaluating quality to determine if a monoclonal antibody has scrambled DSBs. In this webinar, experts will demonstrate the latest hardware and software available to automatically detect, analyze, and evaluate DSBs within monoclonal antibodies.
Sponsored by Bruker
On Demand
Gain new insights on the development of liquid chromatography methods, which are optimized to meet the requirements of modern biotherapeutics characterization laboratories. Emphasis will be given in guiding the selection of the proper column characteristics and instrument settings with respect to biotherapeutic characterization.
Sponsored by Thermo Fisher Scientific
more webcasts |
|
INTERPHEX Exhibitor Showcase
 |
Utilizing the strong synergies in bioreactor technology and polymer manufacturing, Eppendorf has emerged as a global player in the bioprocess marketplace. With a comprehensive offering of single-use and traditional products for the growth of cells and microorganisms, and working volumes of 60 mL – 2,400 L, the Eppendorf bioprocess portfolio can satisfy the demands of process development through production.
INTERPHEX Booth # 3432
/ Learn more / |
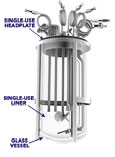 |
You don't need to make a large capital investment to convert your existing benchtop glass bioreactor to a single-use bioreactor. Simply remove your existing headplate and place the preassembled and sterile Distek BIOne System into the glass vessel. The "patent pending" bioreactor liner will mold to and mimic your existing glass system allowing you to continue using your existing cabinet, probes, motor, heating blanket, water jacket and recipes. Since the liner is identical to the dimensions and aspect ratio of your existing vessel, there is no need to change your process. All materials are USP Class VI, animal derivative free and utilized in existing single-use products.
INTERPHEX Booth # 2735
/ Learn more / |
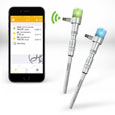 |
Hamilton's intelligent Arc sensors can now be securely monitored, configured, and calibrated from your Bluetooth-enabled device. The unique ArcAir app includes protected user levels and a GMP compliance package for Android and iOS. The Arc system of online sensors eliminates costly, bulky transmitters. ArcAir is compatible with Hamilton's pH, Dissolved Oxygen, ORP, and Conductivity Arc sensors.
INTERPHEX Booth # 3465
/ Learn more / |
|
|
|
on biopharm tv
Collaborative Success Strategies for Biopharm Companies
The Human Element in Pharma Manufacturing
Resolving Drug Shortages
|
|
|
Events
April 25-29, 2016
May 5-7, 2016
June 6-8, 2016
more events |
|
|
Reference Books
 |
Quality by Design (QbD) compiles the best content from BioPharm International and Pharmaceutical Technology to provide valuable insight into the topic and assist in making the business case for QbD, based on the criteria decision makers need to evaluate new initiatives and related technology.
/ Learn more /
More Reference Books |
|
|
|
eBook
 |
Features in this special e-book supplement examine market dynamics, emerging technologies, and regulatory policy that is shaping the future of bioprocessing.
more ebooks |
|
|
|
|
|